As an experienced brewer, I have spent countless hours experimenting with various ingredients to create the perfect concoction.
One question that often comes up in these brewing adventures is whether or not sugar dissolves in alcohol. In this blog post, I will explore this question in depth, drawing from my personal experiences, as well as scientific facts and research.
The short answer is yes, sugar does dissolve in alcohol. However, not as well as it dissolves in water and the process and effectiveness of this dissolution depend on various factors, such as the type of sugar, the type and strength (water content) of alcohol, and the temperature at which the dissolution takes place.
Let’s dive into these factors in more detail, as well as explore some related questions and facts about sugar and alcohol.
The Science Behind Sugar Dissolving in Alcohol
To understand the process of sugar dissolving in alcohol, it’s essential to know the basic principles of solubility. Solubility refers to the ability of a substance (the solute) to dissolve in another substance (the solvent). In this case, sugar is the solute, and alcohol is the solvent.

When a solute dissolves in a solvent, the molecules of the solute are evenly distributed throughout the solvent, forming a homogeneous mixture. This process occurs because the solute molecules interact with the solvent molecules, forming new bonds and breaking old ones.
In the case of sugar and alcohol, the sugar molecules are attracted to the alcohol molecules, and these attractions are strong enough to overcome the bonds holding the sugar molecules together. As a result, the sugar dissolves in the alcohol, forming a homogeneous mixture.
Factors That Affect Sugar Dissolving in Alcohol
Type of Sugar
There are several types of sugar, including sucrose (table sugar), glucose, and fructose, among others. The type of sugar can affect how easily it dissolves in alcohol.
For example, sucrose is less soluble in alcohol than it is in water, which may make it more difficult to dissolve compared to other types of sugar. However, if the alcohol concentration is high enough, or if the sugar is finely powdered, it can still dissolve effectively.
Type of Alcohol
The type of alcohol also plays a role in the dissolution process. Ethanol, the alcohol found in most alcoholic beverages, is a polar solvent, meaning it can dissolve polar solutes like sugar. However, its solubility is limited compared to water due to its lower polarity.
As we will see below, the more water present in the alcoholic solution, the easier it is to dissolve sugar.
Some alcohols, like isopropyl alcohol (rubbing alcohol), are less polar than ethanol and may have a harder time dissolving sugar. In general, the more polar the alcohol, the better it can dissolve sugar.
Temperature
As with most dissolution processes, the temperature can significantly impact the solubility of sugar in alcohol.
Higher temperatures typically increase solubility, allowing more sugar to dissolve in water and alcohol.
However, as seen in the graph below, this effect of the temperature increase on solubility is stronger when ethanol is present.
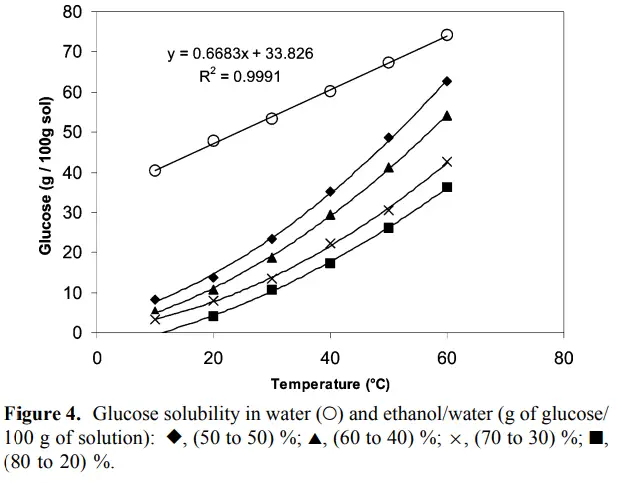
In the graph above, the connection between temperature and solubility is clear. This goes for most things you dissolve, but also for sugar!
Also notice that ethanol is a worse solvent than water and the less the ethanol/water ratio is, the better it dissolves. So mixing in a bit of water means a lot!
Why Dissolve Sugar in Alcohol?
There are several reasons one might want to dissolve sugar in alcohol, especially in brewing and winemaking.
Here are a few examples:
Sweetening Alcoholic Beverages
Many alcoholic beverages, like cocktails and liqueurs, are sweetened with sugar to enhance their flavor. Dissolving sugar in alcohol allows for a homogeneous mixture, ensuring the sweetness is evenly distributed throughout the drink.
Increasing Alcohol Content
In brewing and winemaking, sugar can be added to the fermentation process to increase the alcohol content of the final product. Yeast consumes the dissolved sugar, producing alcohol and carbon dioxide as byproducts. The more sugar that is dissolved and available for the yeast, the higher the alcohol content of the final product.
Balancing Flavor
In some cases, sugar is added to alcoholic beverages to balance out the bitterness or acidity, creating a more complex and enjoyable flavor profile.
Potential Issues with Dissolving Sugar in Alcohol
While sugar can dissolve in alcohol, there are some potential issues to be aware of:
Crystallization
If too much sugar is added to an alcoholic solution, it may not all dissolve, leading to crystallization. This can create a gritty texture in the final product and may be undesirable in some cases.
Impact on Flavor
While sugar can enhance the flavor of alcoholic beverages, adding too much can create an overly sweet and unbalanced flavor. It’s essential to find the right balance to ensure a pleasant final product.
Impact on Alcohol Content
Adding sugar to the fermentation process can increase the alcohol content of the final product. However, if too much sugar is added, it can stress the yeast, leading to off-flavors or even a stalled fermentation.
Alternatives to Dissolving Sugar in Alcohol
If you’re having trouble dissolving sugar in alcohol, or if you want to avoid some of the potential issues listed above, there are alternatives to consider, such as:
Using Different Sweeteners
Honey, maple syrup, agave nectar, and other natural sweeteners can be used in place of sugar. These sweeteners often have a higher solubility in alcohol and can provide unique flavors.
Using Sugar Syrups
As mentioned in my personal experiment, dissolving sugar in a small amount of water to create a syrup can improve its solubility in alcohol. This is especially helpful when using sucrose, which has limited solubility in alcohol.
Conclusion: Sugar Does Dissolve in Alcohol
In conclusion, sugar does dissolve in alcohol, but the effectiveness of this dissolution depends on factors like the type of sugar, the type of alcohol, and the temperature. Understanding these factors can help you achieve the desired results, whether you’re brewing beer, making wine, or crafting cocktails.
Here are ten facts about sugar and alcohol to take away from this discussion:
1. Sugar does dissolve in alcohol.
2. The solubility of sugar in alcohol depends on the type of sugar, the type of alcohol, and the temperature.
3. Ethanol, the alcohol found in most alcoholic beverages, is a polar solvent that can dissolve polar solutes like sugar.
4. Higher temperatures generally increase the solubility of sugar in alcohol.
5. Sugar can be added to alcoholic beverages to enhance flavor, increase alcohol content, or balance bitterness and acidity.
6. Dissolving too much sugar in alcohol can lead to crystallization or an unbalanced flavor.
7. Adding too much sugar during fermentation can stress yeast and lead to off-flavors or stalled fermentation.
8. Alternatives to dissolving sugar in alcohol include using different sweeteners like honey or maple syrup, or using sugar syrups.
9. Sucrose (table sugar) is less soluble in alcohol than other types of sugar, but can still be effectively dissolved with the right conditions.
10. Experimentation and personal preference will play a significant role in finding the right balance of sugar and alcohol for your desired results.
As a homebrewer, I have experimented with dissolving sugar in alcohol on several occasions, particularly when making beer and wine. In one of my experiments, I added sucrose (table sugar) to a batch of homemade red wine to increase its sweetness and balance out the acidity.
I first dissolved the sugar in a small amount of boiling water to create a simple syrup, and then I added this syrup to the wine. The sugar dissolved easily in the water, and as a result, the syrup mixed well with the wine, creating a smooth and homogeneous mixture.
FAQs
Is sugar soluble or solute?
Sugar is a solute, meaning it can dissolve in a solvent such as water.
Why can’t alcohol dissolve sugar?
Alcohol molecules are not polar enough to break apart the polar bonds in sugar molecules, which makes it difficult for them to dissolve sugar.
Is sugar and alcohol soluble or insoluble?
Sugar and alcohol are both soluble in water.
Why is sugar insoluble in alcohol?
Sugar is insoluble in alcohol because it is a polar molecule and alcohol is a non-polar solvent. The polar water molecules in sugar attract each other through hydrogen bonding, making it difficult for them to dissolve in a non-polar solvent like alcohol.
Is sugar soluble or insoluble in water?
Sugar is soluble in water.
Is sugar soluble or not?
Yes, sugar is soluble in water.




