Alcohol is produced by yeast only under conditions with low or no oxygen present in a process known as fermentation. Yeast ferments by consuming sugar and converting it into alcohol and carbon dioxide.
Oxygen is not needed for fermentation. In fact, most organism (including yeast) only ferment in the absence of oxygen.
However, yeast strongly prefers using oxygen to produce energy and grow as it makes the process more than ten times as efficient compared to the anaerobic (without oxygen) process.
When brewing, too much oxygen exposure during the brewing process can lead to oxidation, which can ruin the flavor and aroma of your beer, cider, or wine.
And perhaps more importantly, yeast does not produce alcohol in the presence of oxygen!
That’s why it’s important to understand the role of oxygen in fermentation and how to minimize its exposure during the brewing process.
When oxygen is present, yeast will carry out a different process called cellular respiration, which converts sugar more effectively into energy for the yeast without ethanol as a by-product.
It is much easier for the yeast to use oxygen for energy, so it only ferments when absolutely necessary.
Therefore fermentation will only happen in an environment with low or no oxygen. Oxygen can ruin fermentation because the yeast prefers not to produce alcohol when oxygen is available.
Oxygen also allows other microorganisms like acetic acid bacteria to take hold more easily and spoil your brew.
Oxidation of cider can impact its color, astringency and bitterness, and can reduce the total phenolic compounds present. Oxidation can also combine with other compounds, which can impact the flavor and mouth feel of the cider.
Does Yeast Need Oxygen? Introducing Fermentation Biochemistry
During alcoholic fermentation, yeast produces ethanol (alcohol) as a by-product and carbon dioxide. Yeast cells are able to survive and reproduce using the energy generated from this process in the absence of oxygen – but only if they have to!
In the presence of oxygen, yeast will prioritize using oxygen for cellular respiration to generate energy rather than producing alcohol, which is a less efficient process for the yeast.
That’s why alcohol is only produced by yeast under anaerobic conditions, ensuring that the yeast will produce alcohol instead of using oxygen for cellular respiration.
I am a geek, so I will explain the biochemistry in a bit more detail, but nothing you really need to know as a home brewer. So read on, or skip, depending on your own geekyness!
Fermentation works on the molecular level by converting complex molecules, such as sugars, into simpler molecules, such as alcohol and carbon dioxide.
The process is catalyzed by enzymes, which are proteins that catalyze chemical reactions. The specific enzymes involved in fermentation depend on the type of fermentation taking place.
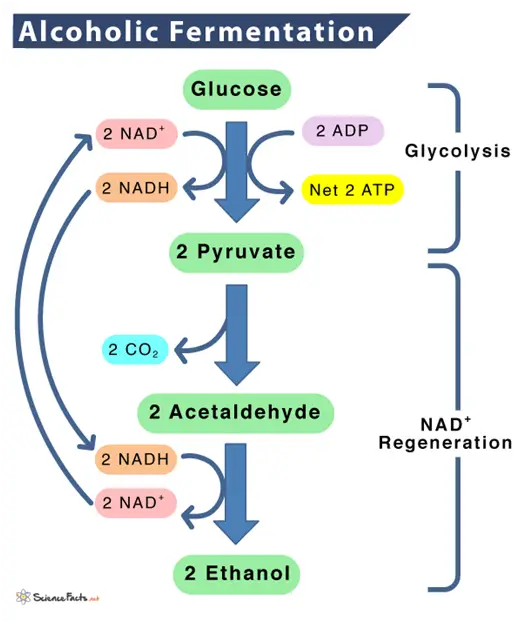
In alcoholic fermentation, yeast produces the enzyme zymase, which converts glucose and fructose into ethanol and carbon dioxide.
The overall chemical reaction can be represented as:
C6H12O6 (glucose/fructose) –> 2C2H5OH (ethanol) + 2CO2 (carbon dioxide) + energy
If you have done malolactic fermentation when brewing, made yoghurt, sour dough, or eaten sauerkraut, you will also have experienced another type of fermentation sometimes relevant in brewing:
Lactic acid fermentation.

In lactic acid fermentation, lactic acid bacteria produce enzymes, which converts glucose into lactic acid and energy. The overall chemical reaction can be represented as:
C6H12O6 (glucose) → 2C3H6O3 (lactic acid) + energy
In both cases, fermentation is a metabolic process that occurs in the absence of oxygen, in anaerobic conditions.
Here’s a simplified overview of how lactic acid fermentation proceeds:
- Glycolysis: The process begins with glycolysis, which occurs in the cytoplasm of the cell. Glycolysis breaks down one molecule of glucose (a six-carbon sugar) into two molecules of pyruvate (a three-carbon compound). This process also generates a small amount of ATP (adenosine triphosphate) and NADH (nicotinamide adenine dinucleotide).
- Formation of Lactic Acid: In the absence of oxygen, the pyruvate generated during glycolysis is converted into lactic acid. This conversion is catalyzed by the enzyme lactate dehydrogenase. The NADH generated during glycolysis donates its electrons to pyruvate, converting it into lactic acid and regenerating NAD+ (nicotinamide adenine dinucleotide), which is necessary for glycolysis to continue.The chemical reaction is as follows:Pyruvate + NADH ↔ Lactic Acid + NAD+
- Regeneration of NAD+: The regeneration of NAD+ is crucial for glycolysis to keep functioning. In the absence of oxygen, cells rely on lactic acid fermentation to regenerate NAD+, allowing glycolysis to continue producing ATP.
- Energy Production: While lactic acid fermentation is less efficient than aerobic respiration in terms of ATP production per glucose molecule, it allows for the rapid generation of ATP when oxygen is limited.
These fermentation processes allow microorganisms such as yeast and lactic acid bacteria to generate energy and reproduce.
But why even make these molecules such as alcohol or lactic acid when they are toxic to the yeast or bacteria at moderate amounts?
Well, they do it because it is the most energetically efficient way of getting rid of the electrons donated by the sugars they eat.
You see, electrons are not nice too keep loose in the cell, so you want to donate them to some molecule.
Normally, under aerobic conditions, oxygen accepts these excess electrons, but when no oxygen is present, some other molecule has to!
The breakdown product of the digested sugars (acetaldehyde) works as an alternative electron acceptor instead of oxygen, but leaves behind a relatively big by-product – ethanol.
Ethanol cannot be broken down further without oxygen, so the yeast will have to live with its own waste product as long as oxygen is absent.
Do yeast cells grow faster with or without oxygen?
Yeast cells grow much faster when oxygen is present. This is why I always recommend stirring your juice or wort well just after adding the yeast, but before the actual primary fermentation has stated.
The graphs below show how the presence of oxygen leads to increased growth rate and sugar consumption or fermentation/respiration rate of different popular yeasts used in wine and cider brewing:
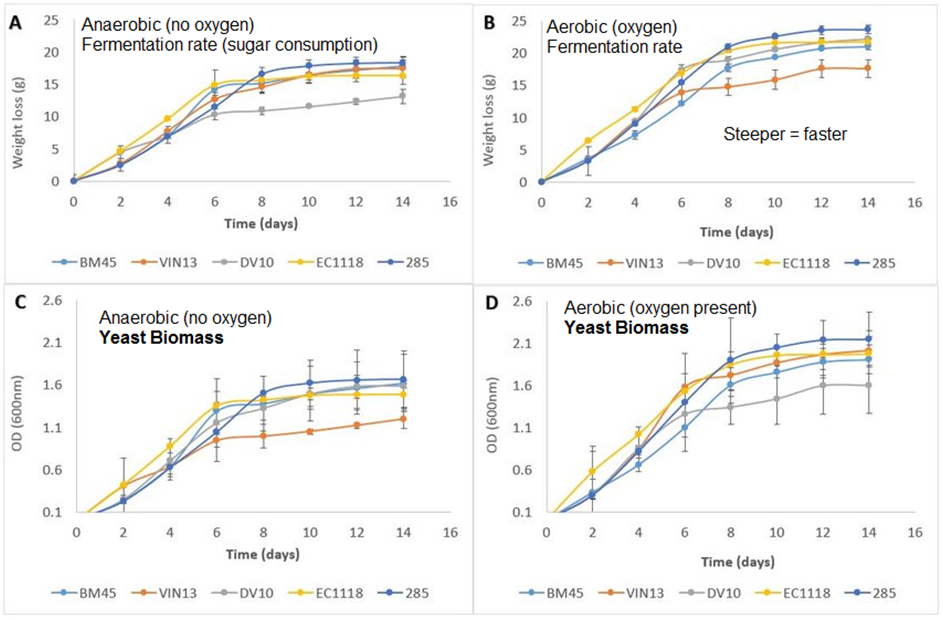
Not only does the yeast work faster when oxygen is present, it also grows faster and can achieve up to twice the total biomass (measured here as OD/optical density of the culture) when oxygen is available.
This is because, as I wrote in the beginning of this article, that aerobic respiration is much more efficient compared to anaerobic respiration.
Fermentation Vs. Respiration: A Molecular Dilemma
Cellular energy production is a fundamental aspect of living organisms, and two primary processes, fermentation and respiration, play pivotal roles in this context. While both pathways involve the breakdown of glucose to generate energy, they differ significantly in their efficiency, dependence on oxygen, and the resulting byproducts.
Fermentation: An Overview
Fermentation is an anaerobic metabolic process that provides cells with a means to generate energy without the involvement of oxygen. Unlike respiration, which utilizes an external electron acceptor in the form of oxygen, fermentation relies on internal mechanisms for the regeneration of the oxidized form of the coenzyme NAD+, essential for sustaining glycolysis.
Key Features of Fermentation
- Absence of External Electron Acceptor: Unlike respiration, fermentation does not require an external electron acceptor like oxygen. Instead, it relies on the internal recycling of electrons within the cell.
- Variety of Byproducts: Fermentation pathways can result in various byproducts depending on the organism and environmental conditions. Common byproducts include organic acids, alcohols, and gases.
- Metabolic Flexibility: Certain microorganisms, such as yeast and bacteria, exhibit metabolic flexibility, allowing them to switch between aerobic respiration and fermentation based on environmental conditions.
Respiration: The Aerobic Advantage
Respiration, both aerobic and anaerobic, is a more efficient process for energy production compared to fermentation. It involves the complete oxidation of glucose, leading to the production of a higher amount of ATP and the efficient regeneration of NAD+ through the electron transport chain.
Key Features of Respiration
- Efficient ATP Production: Respiration yields a higher amount of ATP per glucose molecule compared to fermentation. This efficiency makes it the preferred pathway for energy production when oxygen is available.
- External Electron Acceptors: In respiration, external electron acceptors, such as oxygen or other molecules, facilitate the regeneration of NAD+. This external involvement contributes to the overall efficiency of the process.
- Aerobic and Anaerobic Variants: Organisms capable of respiration can perform either aerobic respiration (in the presence of oxygen) or anaerobic respiration (in the absence of oxygen with alternative electron acceptors).
Does Fermentation Happen in Oxygen-Rich Environments?
While fermentation is traditionally associated with anaerobic conditions, there are instances where organisms may engage in fermentation even in the presence of oxygen. This apparent contradiction can be explained by considering specific advantages conferred by fermentation byproducts.
Adaptive Advantages of Fermentation in Oxygen-Rich Environments
- pH Regulation: Fermentation byproducts, including organic acids, can contribute to the regulation of pH within the cellular environment. This can be crucial for the survival and growth of an organism, even in oxygen-rich conditions.
- Ethanol Production as a Defense Mechanism: Yeast, a common fermenting organism, produces ethanol as a byproduct. Ethanol has antiseptic properties and can act as a natural preservative. By producing ethanol, yeast can inhibit the growth of potential competitors, gaining a competitive advantage in the oxygen-rich surroundings.
- Redox Balance: Fermentation pathways often involve the transfer of electrons, contributing to the balance of cellular redox reactions. This internal cycling of electrons helps maintain redox equilibrium within the cell.
- Survival during Stress Conditions: Fermentation pathways might be more robust or activated under stress conditions, such as nutrient limitations, rapid changes in environmental conditions, or high population densities.
- Niche Adaptation: The ability to switch between aerobic respiration and fermentation provides metabolic flexibility, allowing organisms to adapt to changing oxygen levels in their ecological niche.
Redox Balance: Fermentation vs. Respiration
One critical aspect of cellular metabolism is maintaining the redox balance, ensuring that there is an equilibrium between oxidized and reduced forms of molecules within the cell. Both fermentation and respiration involve redox reactions, but the mechanisms by which they contribute to redox balance differ.
Redox Balance in Respiration
In cellular respiration, whether aerobic or anaerobic, the electron transport chain plays a central role in the regeneration of NAD+. The final electron acceptor, such as oxygen or an alternative molecule, facilitates the efficient recycling of NADH to NAD+, ensuring the continuation of glycolysis and sustained energy production.
Redox Balance in Fermentation
In contrast, fermentation relies on internal processes for NAD+ regeneration. The electron transport chain is not involved, and instead, fermentation byproducts, often in the form of reduced organic molecules, contribute to redox balance. The continuous cycling of NADH back to NAD+ is crucial for sustaining glycolysis, the initial stage of both respiration and fermentation.
Does Fermentation Yield More NAD+ than Respiration?
While both fermentation and respiration involve the recycling of NAD+, respiration is generally more efficient in this regard. The electron transport chain in respiration allows for the external regeneration of NAD+ through the transfer of electrons to an external electron acceptor, resulting in a higher yield of ATP.
On the other hand, fermentation relies on internal processes for NAD+ regeneration, often involving the reduction of organic molecules. While this allows for the continuation of glycolysis and ATP production, the overall efficiency is lower compared to respiration.
Fermentation in Oxygen-Rich Environments: Adaptive Advantages
In the previous section, we explored the adaptive advantages of fermentation in oxygen-rich environments. Now, we’ll delve deeper into specific scenarios where fermentation can be advantageous, even when an ample supply of oxygen is present.
pH Regulation and Fermentation
One notable advantage of fermentation in oxygen-rich circumstances is its role in pH regulation. Fermentation byproducts, including organic acids, can act as pH regulators within the cellular environment. This is particularly important because the internal pH of a cell influences various biochemical processes.
In situations where the external environment is oxygen-rich, but the internal conditions within a cell experience fluctuations or stress, fermentation pathways can be activated to produce organic acids. These acids help in maintaining the optimal pH for cellular functions, ensuring the stability of enzyme activities and overall cell viability.
Ethanol Production as a Defense Mechanism
Certain microorganisms, such as yeast, exhibit the ability to produce ethanol as a byproduct of fermentation, even when oxygen is available. This seemingly counterintuitive behavior can be explained by the defensive properties of ethanol.

In environments where competition with other microorganisms is fierce, yeast might engage in ethanol fermentation as a strategy to create an environment less conducive to the growth of competing species. Ethanol has antiseptic properties and can act as a natural preservative.
By producing ethanol, yeast can inhibit the growth of potential competitors, gaining a competitive advantage in the oxygen-rich surroundings.
Redox Balance in Fermentation: A Closer Look
The redox balance within a cell is critical for maintaining the proper functioning of metabolic pathways. Fermentation, despite its lower energy yield compared to respiration, plays a significant role in redox balance through internal processes.
Internal Recycling of Electrons
In fermentation, the electron transport chain, a prominent feature of respiration, is bypassed. Instead, fermentation involves the internal recycling of electrons within the cell. This internal cycling is facilitated by the reduction of organic molecules, often derived from the breakdown of glucose.
The continuous cycling of NADH back to NAD+ is essential for sustaining glycolysis, the initial step in both respiration and fermentation. While respiration efficiently achieves this through external electron acceptors, fermentation achieves redox balance through the production of reduced byproducts.
Variety of Fermentation Byproducts
Fermentation pathways lead to the production of a variety of byproducts, including organic acids, alcohols, and gases. These byproducts not only contribute to redox balance but also serve other functional roles within the cell and its environment.
For example, lactic acid fermentation involves the reduction of pyruvate to lactic acid, accompanied by the transfer of electrons. This reduction reaction not only regenerates NAD+ but also results in the production of lactic acid, which can influence the acidity of the cellular environment.
Metabolic Flexibility and Niche Adaptation
One of the intriguing aspects of fermentation is the metabolic flexibility exhibited by certain microorganisms. Some organisms, such as yeast and bacteria, can switch between aerobic respiration and fermentation based on environmental conditions. This adaptability allows them to thrive in diverse ecological niches with varying oxygen levels.
Survival in Fluctuating Environments
In environments where oxygen levels fluctuate, organisms with metabolic flexibility have a distinct advantage. They can seamlessly transition between respiration and fermentation, ensuring energy production and survival even when oxygen availability is unpredictable.
Niche-Specific Adaptations
The ability to choose between respiration and fermentation based on environmental factors contributes to niche-specific adaptations. Microorganisms inhabiting diverse ecosystems, from the human gut to deep-sea environments, can optimize their energy production strategies according to the unique conditions of their respective niches.
Redox Balance: Fermentation vs. Respiration
In the previous sections, we explored the redox balance in both fermentation and respiration, highlighting the differences in how these processes contribute to the maintenance of cellular redox equilibrium. Now, let’s delve deeper into the mechanisms by which redox balance is achieved in each pathway and how they influence overall cellular function.
Redox Balance in Respiration: Harnessing External Electron Acceptors
Cellular respiration, whether aerobic or anaerobic, involves the use of an external electron acceptor in the electron transport chain. The final step of the chain transfers electrons to this acceptor, allowing the regeneration of NAD+ and ensuring the continuity of glycolysis.
The Electron Transport Chain
- Aerobic Respiration: In aerobic respiration, the final electron acceptor is oxygen. The electron transport chain efficiently transfers electrons through a series of protein complexes, leading to the formation of a proton gradient across the mitochondrial membrane.
- Anaerobic Respiration: In anaerobic respiration, alternative electron acceptors, such as nitrate or sulfate, are utilized. While the process is less efficient than aerobic respiration, it still allows for the regeneration of NAD+.
NADH Recycling
The key advantage of respiration lies in the external regeneration of NAD+. The electron transport chain actively pumps protons across the membrane, creating a proton motive force that drives the regeneration of NAD+.
Redox Balance in Fermentation: Internal Recycling of Electrons
In contrast to respiration, fermentation bypasses the electron transport chain and relies on internal processes for the recycling of NAD+. The absence of an external electron acceptor necessitates alternative mechanisms for maintaining redox balance.
Internal Cycling of NADH
- Fermentation Pathways: Fermentation involves the reduction of organic molecules, often derived from glycolysis, to produce byproducts like organic acids or alcohols. These byproducts serve as both end products of fermentation and vehicles for the internal recycling of NADH.
- Role of Fermentation Byproducts: By reducing pyruvate to fermentation byproducts, such as lactic acid or ethanol, the cell regenerates NAD+. This internal cycling of electrons allows glycolysis to continue, providing a source of ATP in the absence of an external electron acceptor.
Efficiency Considerations: Fermentation vs. Respiration
While fermentation plays a crucial role in maintaining redox balance, especially in anaerobic conditions or oxygen-limited environments, it is generally less efficient than respiration in terms of ATP production.
ATP Yield in Fermentation
Fermentation pathways yield fewer ATP molecules per glucose molecule compared to respiration. The absence of an electron transport chain limits the overall energy extraction from the substrate, making fermentation a less favorable option when oxygen is abundant.
ATP Yield in Respiration
Respiration, with its reliance on an electron transport chain and external electron acceptors, allows for a more extensive extraction of energy from glucose. This results in a higher ATP yield and provides a substantial advantage in terms of cellular energy production.
Fermentation vs respiration in summary
In this comprehensive exploration of fermentation and respiration, we’ve delved into the intricacies of these two fundamental cellular processes.
From the adaptive advantages of fermentation in oxygen-rich environments to the redox balance mechanisms employed by both pathways, the intricate dance of metabolic reactions within cells is essential for their survival and function.
The dynamic interplay between fermentation and respiration underscores the incredible diversity and adaptability of life at the cellular level. Microorganisms, through evolutionary processes, have developed strategies to thrive in a wide range of environments, from oxygen-rich to oxygen-limited niches.
As we continue to unravel the mysteries of cellular metabolism, the intricate dance of metabolic pathways and the strategic use of fermentation and respiration showcase the resilience of life and its ability to persist in ever-changing conditions.
In conclusion, the coexistence of fermentation and respiration highlights the versatility of cellular metabolism and the remarkable ways in which organisms navigate the challenges of their environments.
Overall Conclusion
It is important to understand the role of oxygen and how it affects fermentation in order to produce quality beer, cider or wine.
Fermentation occurs in anaerobic conditions and oxygen can ruin brewing of especially wine, cider and beer by encouraging the growth of unwanted microorganisms, leading to off-flavors, spoilage and direct oxidation of alcohol into acetic acid.
Therefore, oxygen should be limited during the brewing process and appropriate bottling techniques should be used to minimize oxygen exposure during aging.




-recipe.jpg)